- The objective of this post is to provide detailed good practices in sampling area including gowning at entrance and exiting the sampling area and recording of essential data of sampling booths and rooms and to describe cleaning procedures of sampling booths and sampling rooms for sampling of raw, packaging material and their retained samples.
- Description of Sampling Area:
- Sampling area of Pharmaceutical Ingredients in Hormone sites is consisting of two areas one for active ingredients and another one for inactive ingredients these two areas mainly consist of the following rooms:
- Personnel Air Lock Inlet (PAL In).
- Secondary Gowning Room.
- Washing Room.
- Sampling Room.
- Sampling Booth.
- Material Air Lock.
- Air Shower Room.
- Personnel Air Lock Outlet (PAL Out).
- Sampling area of Pharmaceutical Ingredients in Aerosol site consists of the following rooms for both active and inactive ingredients:
- Personnel Air Lock Inlet (PAL In).
- Secondary Gowning Room.
- Washing Room.
- Inactive Sampling Room.
- Inactive Sampling Booth.
- Material Air Lock.
- Personnel Air Lock Outlet (PAL Out).
- Entry of personnel to the Sampling Area:
- Only authorized personnel (according to authorization list) from the QC department are allowed for entrance of sampling area.
- Entrance of Sampling Area is done through the (PAL In) rooms.
- Wash your hand with hand sanitizer and dry them.
- In case of Hormone sampling area enter the secondary gowning room.
- In case of Hormone sampling area, wear the secondary gowning garments for the sampling of active ingredients which is dedicated for the sampling area or the disposable overall, overhead, over beard and the dedicated shoes or the overshoes
- In case of active hormone material sampling, make sure garments are changed after sampling of each active raw material and disposed in laundry bag till cleaned and washed off later.
- Enter the corridor.
- Enter the Sampling room.
- Transfer of materials into and out of the Sampling Area:
- Materials are transferred to and from sampling area through material airlock (MAL).
- Clean the outer surface of the containers using vacuum cleaner and wiping with towels (if necessary) before entry and exit of containers to prevent introduction of dust into and out of sampling room.
- Recording of Rooms temperature / Relative humidity and differential pressures:
- Before starting Sampling, record all rooms temperature and relative humidity using a calibrated portable Thermo-hygrometer and record differential pressures between rooms using calibrated differential pressure gauge
- Ensure that at PALs, MAL and sampling corridor are bubble airlocks (positive) to the adjacent
- Ensure that washing room and sampling room are negative to the corridor.
- Cleaning and disinfection of Sampling Booth:
- After sampling booth usage, clean by wiping the sampling booth stainless steel walls internal and external, balance, balance table, curtains and floor with a lint free towel and wipers soaked in detergent then with purified water 3 times then dry with a dry lint free towel, then wipe all above items with the disinfectant and spray and let to dry for a specified time for each disinfectant by the QC microbiological Lab.
- Note: Disinfection is done before and after use.
- Replace the disinfectant type every 7 days. (Rotate between 2 or 3 types of disinfectant)
- Record all cleaning activities of the sampling booth
- Cleaning and disinfections of Sampling Area Rooms:
- All sampling rooms shall be cleaned and disinfectant daily (if used).
- Clean floors, door, walls, and lockers by wiping with a detergent then by purified water then drying.
- Disinfect using a disinfectant by wiping then spraying of all above items then let them to dry for a specified time as per recommendations of the QC Microbiological lab
- All wipes and towels used in cleaning of active material sampling area should be disposed in a red hazard bag and handled properly
- Record all cleaning activities of sampling area rooms
- Sampling Facilities:
- Sampling facilities are designed to:
- Prevent contamination of opened container, the materials, and the sampler.
- Prevent cross-contamination by other materials, products and the environment.
- Protect samplers during sampling procedure.
- This is achieved through maintenance of differential pressure between rooms, sampling under qualified weighting booth and proper gowning procedures(Sampling garment with hood.
- Sampling facilities are designed to:
- Preparation for Sampling:
- Sampling tools:
- The QC sampler should have all tools needed for sampling process:
- Sampling tools:
- Sampling area of Pharmaceutical Ingredients in Hormone sites is consisting of two areas one for active ingredients and another one for inactive ingredients these two areas mainly consist of the following rooms:
- Stainless Steel spatulas dedicated for each material( sterile or Depyrogenated in case of microbiological testing)
- Butter paper.
- Sampling Labels.
- Polyethylene bags
- Clean lint free towel.
- Sampling containers: –
- Polyethylene bags for raw materials (for chemical tests)
- Covered amber glass bottles for light-sensitive liquid raw materials
- Sterile containers for Microbiological testing
- Depyrogenated glass containers for endotoxin testing.
- Description of Sampling Area:
- Special precautions:
- Care must be taken when sampling acids, caustics, etc… & MSDS must be checked before sampling of chemicals to achieve safety.
- PPE must be worn before sampling.
- Check all containers have supplier lot number (on supplier label) as the attached supplier certificates of analysis or forms.
- Check the appearance of the drum & its contents before sampling & note any abnormal appearance & report to your supervisor immediately.
- Hygroscopic material should be sampled as quickly as possible.
- Containers of raw materials shall be opened for sampling in the sampling rooms inside sampling booth to avoid contamination of the material or any deterioration in its quality.
- Special care is necessary when resealing sampled containers to prevent damage or contamination of raw materials.
- Special care is necessary when sampling Hormone active materials. The sampler should wear clean sampling garment with hood connected to compressed air which should be changed between successive sampling procedures of active raw material.
-
- Cleaning of sampling tools
- All sampling tools (spatula) for active material should be dedicated for each active material with unique code index
- The code consisting of
- Cleaning of sampling tools
YY-00X
Where:
| YY | AM (Active material) |
| 00X | Serial |
- Once used, the sampling tools should be rinsed thoroughly with running purified water three times (for at least 2 minutes) until visually clean.
- Drying: Finally dry the sampling tools by compressed air at room temperature, spray ethanol 70% then put it in Polyethylene bags and seal and store in dry clean place in tools locker at room temperature.
- In case of oils, soak in ethanol 96% for 10-15 minutes then rinse thoroughly with running purified water three times (for at least 2 minutes) until visually clean then dry with compressed air before sealing in labelled polyethylene bag.
- Stick Clean label in sampling tools (including cleaning date and time and valid hold time)
- Cleaning of Sampling garment with hood
- QC sampler shall take off the hood after sampling process is done and put it in laundry bag.
- QC sampler shall take off the garment after pass through air shower room and put it in laundry bag.
- Garment with hood shall be change between each active sampling to prevent cross-contamination.
- Sampling Procedures
- QC sampler shall check ERP system daily for any new received material to be sampled
- QC Sampler who is dealing with materials containers should enter sampling room through the gowning room (personnel flow).
- White coats and trousers or garment with hood kept on shelves shall be put on before entering sampling room.
- Mist or dust mask, powder-free disposable gloves and over heads should be worn during the sampling process, and should be changed between each raw material.
- The material should be brought to the sampling room from the specified door of the material (material air lock) and cleaned with vacuum cleaner.
- Before sampling ensure that the sampling room doors are well closed.
- The sampling room must be locked all the time during sampling procedure
- Only current logbook should be available in the sampling room.
- Switch on the sampling booth (LAF), wait for 15 minutes before beginning of sampling activities and record it in Logbook
- After finishing of sampling process, transfer the sampling tools in polyethylene bag to be washed in washing area
- Raw Materials:
- Identification test is 100% for each new consignment.
- Assuming that the raw material is uniform, and received from recognized source, the (n plan) can be used as following:
- Samples can be withdrawn from any part of the container.
- This plan is based on the formula:
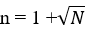
Original samples are where:
N= the total number of sampling units.
n= Number of sampling obtained (rounded to the next largest integer).
- Taken from n sampling units randomly and these are placed in separate sample containers, visually inspected for appearance and identity of each of these samples. If the results are concordant, the original samples are combined into final composite sample from which an analytical sample is prepared for full testing.
- The amount of each sample should be sufficient for doing the all required tests.
- Quantity sheet for active material
Table 1: Values of n for The N sampling units for n plan
| Values of N | Values of n |
| 1 | 1 |
| 2 | 2 |
| 3 – 4 | 3 |
| 5 – 7 | 4 |
| 8 – 11 | 5 |
| 12 – 16 | 6 |
| 17 – 22 | 7 |
| 23 – 28 | 8 |
| 29 – 36 | 9 |
| 37 – 44 | 10 |
- Packaging Materials:
- According to AQL
- Labelling:
- Sampling and labelling procedures should be supervised by raw material and packaging material supervisor and/or his designee and sign
- Sign and Stick “Quarantine” Label on each drum
- In case of sterile material, the QC sampler should stick conditioning release label without sampling till it is sampled on-line.
- For non-sterile material Prepare a composite sample in a suitable properly identified container keeping the rest of the individual samples for individual tests (as physical tests, identity, and other designated tests).
- Record all data in sampling log book in annex:
- sampling Active , In-Active , sterile hormone and Aerosol Site
- Sign and Stick “Sampled” label beside the supplier label on each sampled drum demonstrating the number of each sampled container
- Sign and Stick “Sample” label with the required data on the relevant sampling containers
- Send the sample to Q.C to analysis. Certificate of analysis will submitted to quality assurance to take the final decision. Upon release to specification sign and stick” Release” label on each drum. If non-conformance to the desired specs was found out, the “Reject” label should be signed and affixed
- Exiting the Sampling Hormone Area:
- Exit the sampling area through the air shower room.
- Stand in front the air shower for specific air shower operating time then exit the air shower room to the PAL Out room.
- Remove the secondary gowning and wear the primary gowning again and exit to the warehouse corridor.
- Exiting the Sampling Aerosol Area:
- Exit the sampling area through exit gowning room.
- Remove the gowning, store in lockers exit to the warehouse corridor