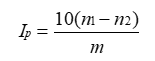-
Acid value:
- Preparation of the reagents:
- Preparation 0.1 M sodium hydroxide: Dissolve 4.2 g of sodium hydroxide in sufficient carbon dioxide-free water to produce 1000 mL.
- Preparation of Phenolphthalein solution: A 1.0% w/v solution of phenolphthalein in ethanol (96%).
- Procedure:
- The acid value IA is the number that expresses in milligrams the quantity of potassium hydroxide required to neutralize the free acids present in 1 g of the substance.
- Dissolve 10.00 g of the substance to be examined, or the quantity prescribed (mg) in 50 ml of a mixture of equal volumes of alcohol R and ether R, previously neutralized with 0.1 M potassium hydroxide, unless otherwise specified, using 0.5 ml of phenolphthalein solution as indicator. When the substance to be examined has dissolved, titrate with 0.1 M potassium hydroxide until the pink color persists for at least 15 s
- (End point (n)= ml of 0.1 M potassium hydroxide).
- m= weight of the substance in g
- Preparation of the reagents:
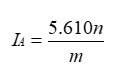
-
-
Peroxide Value:
-
The peroxide value IP is the number that expresses in mill equivalents of active oxygen the quantity of peroxide contained in 1000 g of the substance as determined by the methods described below. When the monograph does not specify which method is to be used, method A should be applied. Any change from method A to method B should be validated.
- Preparation of the reagents:
- Starch Solution: Triturate 1.0 g of soluble starch with 5 mL of water and whilst stirring pour the mixture into 100 mL of boiling water containing 10 mg of mercuric iodide.
- 01 M sodium thiosulphate: Dissolve 2.5g sodium thiosulphate and 20mg of sodium carbonate in 1000ml water.
- Procedure:
- Place 3.00 g of the substance to be examined in a 250 ml conical flask fitted with a ground-glass stopper. Add 15 ml of a chloroform of 30 mL of glacial acetic acid. Shake to dissolve the substance and Add 1 mL of a freshly prepared solution of 1.3 g of potassium iodide in 1 mL of water.
- Shake for exactly 1 min and set aside in the dark for 3 minutes then add 100 ml of water R. Titrate with 0.01 M sodium thiosulphate adding the titrant slowly with continuous shaking until the yellow color is almost discharged. Add 5 ml of starch solution R and continue the titration, shaking vigorously, until the color is discharged (n1 ml of 0.01 M sodium thiosulphate).
- Carry out a blank test under the same conditions (n2 ml of 0.01 M sodium thiosulphate). The volume of 0.01 M sodium thiosulphate used in the blank titration must not exceed 0.1 ml.