Objective:
-
- To establish a procedure for swab and rinse sampling for recovery determination of test surface to evaluate cleaning efficacy
- Determination and calculation of recovery factor for APIs and detergents in cleaning validation of pharmaceutical manufacturing equipment.
- Setting methods for reporting and evaluation of samples withdrawn in cleaning validation.
Procedure:
-
- Recovery factor determination approach:
- Upon taking a sample from the equipment with the cotton swab or rinse, the entire API and/or detergent associated with the equipment is not removed by the swab/rinse and this error of recovery is managed by the recovery factor during the calculations in cleaning validation.
- Recovery steps:
- A solution of known concentration of API / detergent is applied/spiked to coupons or plates of 25 cm2 representing the cleaned equipment surfaces (stainless steel, glass, silicon …etc.). Allow the plate to air dry
- For swabbing, swab the plate with the cotton swab and dissolve in a suitable known predetermined volume of diluent.
- For rinsing, place the coupon at a slant over a clean glass beaker. Pipette a predetermined volume of the diluent on the spiked plate area, allow the rinse water to cascade down the coupon covering the whole spiked area and receive it in the container for further analysis.
- Diluents should be prepared on the basis of the solubility of the active ingredient / detergent. Shake/Sonicate the cotton swab and diluents for 5 minutes to dissolve the content properly. Makeup the solution with the diluents, if needed, to get the desired concentration as the standard solution is prepared.
- Mix the solution properly and analyze as per the method of analysis. Calculate recovery factor by the following recovery factor formula:
- Recovery factor determination approach:
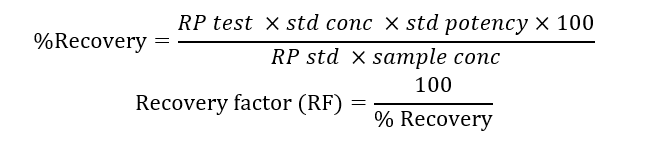
Where
RP test: response due to swab or rinse sample solution
RP std: Response due to standard solution
Std conc: weight of standard in dilution solvent (mg/ml)
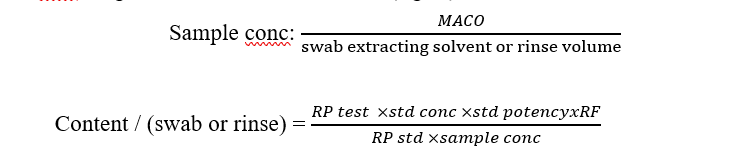
Std conc.: weight of standard in dilution solvent (mg/ml)
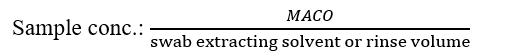
- The previous steps are repeated for at least three times, where the average recovery factor for each surface is determined and reported
- Upon cleaning samples analysis, the calculated results of the sample are multiplied by the recovery factor to report the actual results.
- Criteria to choose the mostly suitable swab for cleaning and recovery determination:
To get the accurate results, it is required to use the mostly suitable swab to take the sample because swab is the main component that has the highest chances of error and may result in the inaccurate cleaning validation and recovery calculation as follows:
- Minimal residues interferences:
The contaminants extracted from the swab during its use should be the minimum. These are the contaminants in the blank swab. Appropriate blanks and controls should be utilized to assure that the recovery percentages calculated are reflective of the recoveries achieved in actual swab sampling of equipment
- High recovery rate:
Recovery rate is the percentage of the released amount of the sampled content. A swab doesn’t release whole swabbed content but it should release the maximum that it can. Minimum 60 % recovery rate is acceptable but the higher recovery rate is better.
- Solvent compatibility:
A variety of solvents are used for swab sampling according to the residues of the previous products. Therefore, the swab must not have any chemical reaction with the solvent used for sampling as this may alter the actual cleaning validation and recovery results. Therefore, swabs must be compatible especially with the solvent to be used for the swabbing.
- Low particle generation:
Swabs may shed particles during its use on equipment surface and it is difficult to find a non-particle shedding swab at all. Low particle generating swabs should be selected. Solvents should also be filtered after extraction and before being analyzed especially if the used method is a chromatographic based one
- Criteria for correct rinse sampling for recovery determination
- The solvent used for the recovery study should be of the same quality and temperature as that used in the cleaning validation protocol for rinse sampling.
- The ratio of the volume of solvent used to the surface area rinsed should be nearly the same as the ratio used in the cleaning protocol
- The time of contact of the solvent in the recovery study should be the same.
- Appropriate blanks and controls should be utilized to assure that the recovery percentages calculated are reflective of the recoveries achieved in actual rinse sampling of equipment