How to perform uniformity of mass of single dose preparation of different dosage forms.
-
Uniformity of dosage units:
- Dosage units are defined as dosage forms containing a single dose or a part of a dose of an active substance in each dosage unit. The uniformity of dosage units’ specification is not intended to apply to suspensions, emulsions, or gels in single-dose containers intended for cutaneous administration.
- The term “Uniformity of dosage unit” is defined as the degree of uniformity in the amount of the active substance among dosage units. Therefore, the requirements of this document apply to each active substance being comprised in dosage units containing one or more active substances, unless otherwise specified. The uniformity of dosage units can be demonstrated by either of 2 methods: content uniformity or mass variation
- The test for content uniformity of preparations presented in dosage units is based on the assay of the individual contents of active substance (s) of a number of dosage units to determine whether the individual contents are within the limits set. The content uniformity method may be applied in all cases.
-
- The test for mass variation is applicable for the following dosage forms:
- Solutions enclosed in single-dose containers and in soft capsules.
- Solids (including powders, granules and sterile solids) that are packaged in single-dose containers and contain active or inactive added substances.
- Solids (including sterile solids) that are packaged in single-dose containers, with or without active or inactive added substances, that have been prepared from true solutions and freeze-dried in the final containers and are labelled to indicate this method of preparation.
- Hard capsules, uncoated tablets, or film-coated tablets, containing 25 mg or more of an active substance comprising 25 present or more, by mass, of the dosage unit or, in the case of hard capsules, the capsule contents, except that uniformity of other active substances present in lesser proportions is demonstrated by meeting content uniformity requirements.
- The test for content uniformity is required for all dosage forms not meeting the above conditions for the mass variation test.
- Alternatively, products that do not meet the 25 mg/ 25 per cent threshold limit may be tested for uniformity of dosage units by mass variation instead of the content uniformity test on the following condition:
- The concentration Relative Standard Deviation (RSD) of the active substance in the final dosage units is not more than 2 per cent, based on process validation data and development data, and if there has been regulatory approval of such a change. The concentration RSD is the RSD of the concentration per dosage unit (m/m or m/v) where concentration per dosage unit equals the assay result per dosage unit divided by the individual dosage unit mass
- The test for mass variation is applicable for the following dosage forms:
-
Content uniformity:
Select not less than 30 units, and proceed as follows for the dosage form designated. Where different procedures are used for assay of the preparation and for the content uniformity test, it may be necessary to establish a correction factor to be applied to the results of the latter.
- Solid dosage forms:
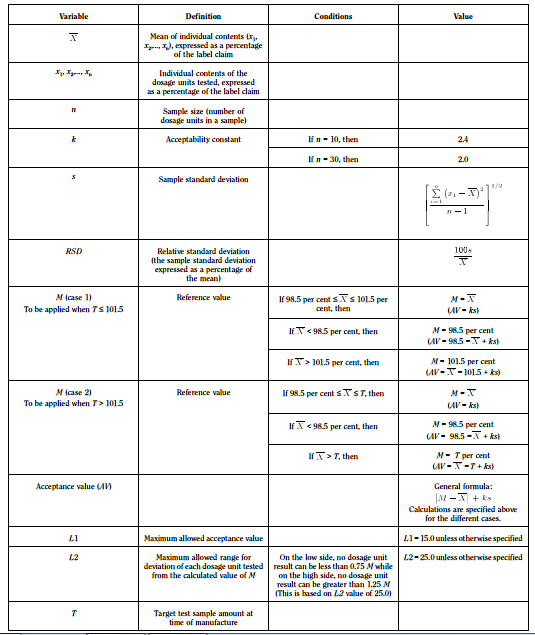
- Assay 10 units individually using an appropriate analytical method. Calculate the acceptance value
- Liquid dosage forms:
- Assay 10 units individually using an appropriate analytical method. Carry out the assay on the amount of well-mixed material that is removed from an individual container in conditions of normal use. Express the results as delivered dose. Calculate the acceptance value
- Calculation of Acceptance Value:
- Calculate the Acceptance Value (AV) using the formula:
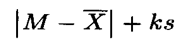
In which the terms are as following:
-
Mass variation:
Carry out an assay for the active substance (s) on a representative sample of the batch using an appropriate analytical method. This value is result A, expressed as percentage of label claim (see Calculation of Acceptance Value). Assume that the concentration (mass of active substance per mass of dosage unit) is uniform. Select not less than 30 dosage units, and proceed as follows for the dosage form designated.
- Uncoated or film-coated tablets:
Accurately weigh 10 tablets individually. Calculate the active substance content, expressed as percentage of label claim, of each tablet from the mass of the individual tablets and the result of the assay. Calculate the acceptance value.
Calculation of Acceptance Value:
Calculate the acceptance value (AV) as shown in content uniformity, except that the individual contents of the units are replaced with the individual estimated contents defined below.
X1, X2, …….., Xn = individual estimated contents of the dosage units tested
Where:
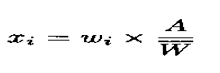
W1, W2, …………., Wn = individual masses of the dosage units tested,
A = content of active substance (percentage of label claim) obtained using an appropriate analytical method,
= mean of individual masses (W1, W2, …………., Wn ).
- Criteria:
Apply the following criteria, unless otherwise specified:
- Solid and liquid dosage forms:
- The requirements for dosage uniformity are met if the acceptance value of the first 10 dosage units is less than or equal to L1.
- If the acceptance value is greater than L1, test the next 20 dosage units and calculate the acceptance value. The requirements are met if the final acceptance value of the 30 dosage units is less than or equal to L1 and no individual content of the dosage unit is less than (1 — L2 x 0.01) M nor more than (1 + L2 x 0.01) M in calculation of acceptance value under content uniformity or under mass variation. Unless otherwise specified, L1 is 15.0 and L2 is 25.0.